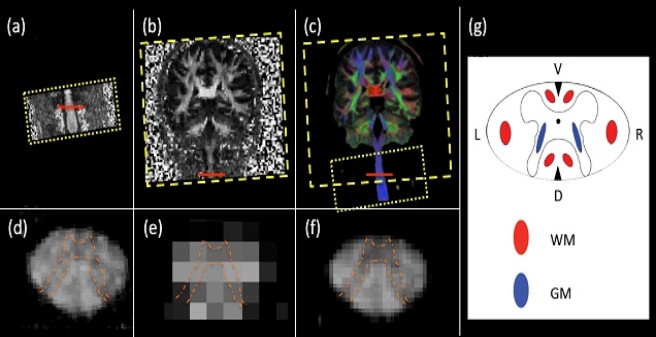
Pilot data from a clinical 3T system illustrating the feasibility of fusing clinical diffusion MRI (dMRI) of spine and brain acquired at different resolution. (a) and (b): Fractional anisotropy (FA) maps of a healthy subject, acquired with different spatial resolution in the spinal cord (a: 0.8x0.8x5.5 mm3) and in the brain (b: 2x2x2 mm3). Both datasets were optimized with respect to time efficiency and resolving the underlying anatomical information for clinical applications. (c): Fusion of (a) and (b) yields a high-quality FA map overlaid by fibre orientation in colors (red: left-right, green: anterior-posterior, blue: head-feet). (d-f) Magnification of the slice in the spinal cord, highlighted by the red line in (a-c), respectively. (g) Schematic drawing of the spinal cord, where the butterfly-shaped grey matter (blue) is surrounded by white matter (red). Note that the in the magnification in (d-f) the characteristic butterfly-shaped grey matter (highlighted by orange dashed line) is more visible after fusion (f) as compared to the original images (d) and (e).
Understanding the mechanisms of atrophy associated with spinal cord injury: the application of MRI-based in vivo histology and ex vivo histology
Spinal cord injuries (SCI) are mainly caused by traumatic events, such as traffic and sports accidents and violence. Paraplegia (legs paralysed) and tetraplegia (both legs and arms paralysed) permanently, severely and dramatically reduce the quality of life of the affected person as well as their ability to remain a member of the workforce. Recent advances in the field of magnetic resonance imaging (MRI) have vastly improved how we can visualize and interrogate the structural organisation and functioning of the central nervous system. However so far, the range of biological changes that may underlie the observed changes cannot be disentangled. This ERA-NET consortium aims to establish the missing link between measured MRI signals and changes in the underlying tissue microstructure, which will help us to explain and better understand the disease processes associated with spinal cord injury.
Our local project will focus on the white matter and bridge the gap between the micro-scale tissue properties and the measured MRI signal within a macroscopic voxel by using biophysical models that exploit symmetries in the organization of fibre pathways (see Fig. 1). Furthermore, we will develop innovative approaches to translate these biophysical models into a clinical setting. This project is tightly linked to the project headed by Nikolaus Weiskopf at the MPI in Leipzig and to the project headed by Martina Callaghan at the WTCN in London .