News
New Publication on Long-Term Immune Responses After MERS Vaccination
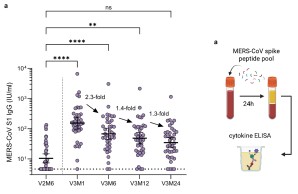
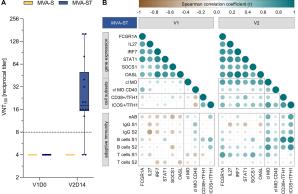
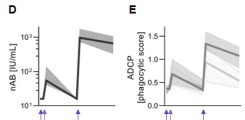
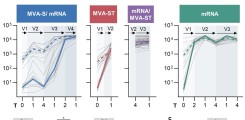
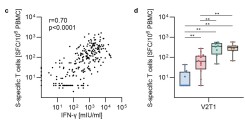
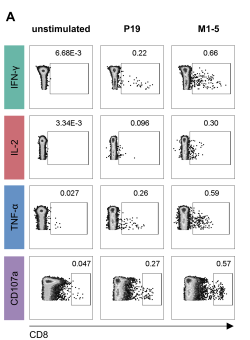
The new year starts with a wonderful publication in a renowned scientific journal reporting sustained immune responses following vaccination with the MERS vaccine candidate MVA-MERS-S. The study demonstrates that MERS-CoV-specific antibody and T cell responses remain detectable for at least two years after the third vaccination, with antibody levels comparable to peak responses and the ability to cross-neutralize spike variants.
The study was funded by the Coalition for Epidemic Preparedness Innovations (CEPI), the German Centre for Infection Research (DZIF), and the German Research Foundation (DFG) through Collaborative Research Centre (CRC) 1648. The work represents a close collaboration between the IIRVD, Charité – Universitätsmedizin Berlin, Philipps-Universität Marburg, and the Erasmus Medical Center Rotterdam.
These findings support the potential of MVA-MERS-S as a promising MERS vaccine candidate and highlight the importance of booster vaccination for long-term immunity.
Congratulations to all authors on this successful and impactful publication!
Publication:
Mayer L, Fathi A, Weichel HM, Raadsen MP, Dahlke C, Mykytyn A, Rodon J, Gerresheim GK, Te Marvelde MR, Weskamm LM, Grewe I, Schlesner C, Lütgehetmann M, Drosten C, Becker S, Haagmans BL, Hardtke S, Addo MM. Two-year persistence of MERS-CoV-specific antibody and T cell responses after MVA-MERS-S vaccination in healthy adults. Nat Commun. 2026 Jan 9;17(1):480. doi: 10.1038/s41467-025-68248-5.
Contact
Leonie Mayer
01/14/2026
The Institute for Infection Research wishes all 'Jecke' a wonderful 2025/2026 carnival season! Alaaf!”
11/11/2025
A Transatlantic Joint-Lab Model for Interdisciplinary Research
Dr. Dr. Angelique Hölzemer, a clinical scientist at the Institute for Infection Research and Vaccine Development, has partnered with Dr. Wilfredo F. Garcia-Beltran from the Ragon Institute at Harvard Medical School to establish a joint German-American laboratory.
Since 2021, this collaboration has linked researchers from Angelique Hölzemer’s Laboratory for Infection and Immune Regulation at the Institute for Infection Research and Vaccine Development (IIRVD) with Wilfredo Garcia-Beltran’s Laboratory for the Investigation of Natural Killer Cells at the Ragon Institute in Boston. Together, they study innate immune mechanisms and viral infections.
Angelique and Wilfredo exemplify how a transatlantic partnership can combine resources, expertise, and diverse research methodologies. Through weekly online meetings, shared protocols, and close cooperation, they have created a highly productive platform for interdisciplinary innovation. Their recent publication in *Nature Microbiology* highlights the significance of this model for the future of collaborative science.
This initiative demonstrates how cross-border partnerships can accelerate research, foster creativity, and promote scientific excellence. The work of Angelique Hölzemer and Wilfredo Garcia-Beltran underscores that international cooperation is essential for addressing emerging viral threats and advancing scientific breakthroughs collectively.
Publication
Hoelzemer A, Garcia-Beltran WF. A transatlantic joint lab model for interdisciplinary research. Nat Microbiol. 2025 Oct;10(10):2356-2358. doi: 10.1038/s41564-025-02127-z.
10/23/2025
New Funding and Publication for Dr. Dr. Ulrike Lange
Immunomodulation via Proviruses in HIV-1 Infections
The Werner Otto Foundation is supporting a one-year research project on the immunomodulatory effects of proviruses in HIV-1 infections. Funding of €72,000 is available for this project. The aim of the research is to investigate how integrated viral genomes influence gene regulation in infected T-cells.
TheraImmun: Innovative Gene Therapy Against HTLV-1
The research project TheraImmun, starting in August 2025, is developing an innovative, modular gene therapy platform for targeted modification of immune cells. The goal is to treat the HTLV-1 virus, which is associated with severe diseases such as T-cell leukemias and neurological disorders and currently lacks specific therapy. The project is funded with a total of €1.7 million, of which €680,000 is allocated to the Leibniz Institute for Virology (LIV).
The approach is based on a designer recombinase (RecHTLV) that removes the viral genetic material from immune cells, complemented by nanobody technology for targeted delivery. Modern 3D organoid models are used for preclinical studies, simulating realistic tissue environments and enabling animal-free research.
Publication
New Insights into Aggressive Brain Tumors in Children: Transposon Activity as a Differentiating Factor
These findings could pave the way for new therapeutic approaches. The analysis of transposable element (TE) activity potentially offers new strategies for targeted treatments, such as immunotherapy. Overall, the results emphasize the importance of molecular characterization of atypical teratoid/rhabdoid tumors (ATRTs) and could lead to more effective, personalized treatment regimens for affected children.
Hamann MV, Godbole S, Adiba M, Leddy SM, Navolić J, Tabatabai G, Merk DJ, Lange UC, Neumann JE. Expression of LTR and LINE1 transposable elements defines atypical teratoid/rhabdoid tumor subtypes.Acta Neuropathol Commun. 2025 Jul 22;13(1):159. doi: 10.1186/s40478-025-02078-w
Contact
Ulrike Lange
08/20/2025
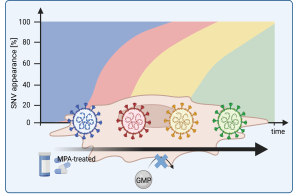
New Publication on the Immunosuppressant Mycophenolic Acid and Its Role in Promoting the Emergence of New SARS-CoV-2 Variants
Researchers from the University Medical Center Hamburg-Eppendorf, Ruhr University Bochum, and the Leibniz Institute of Virology have found that the immunosuppressive drug mycophenolic acid (MPA) may facilitate the emergence of new SARS-CoV-2 variants.
MPA is a well-established antimetabolite commonly used to suppress immune responses in patients undergoing organ transplantation (e.g., kidney, liver, or heart), in order to prevent graft rejection. In addition to its immunosuppressive role, MPA also inhibits the replication of several viruses, including SARS-CoV-2, respiratory syncytial virus (RSV), and monkeypox virus (MPXV). However, the study demonstrates that SARS-CoV-2 can escape this inhibition, suggesting that long-term MPA use could drive viral evolution in immunocompromised individuals.
Publication:
Meister TL, Nocke MK, Heinen N, Burkard TL, Brüggemann Y, Westhoven S, Trüeb B, Ebert N, Thomann L, Lubieniecki KP, Lubieniecka JM, Döring K, Herrmann M, Haid S, Pietschmann T, Wiegmann B, Tao R, Pfefferle S, Addo MM, Thiel V, Drexler I, Babel N, Phuc Nguyen H, Brown RJP, Todt D, Steinmann E, Pfaender S. Mycophenolic acid treatment drives the emergence of novel SARS-CoV-2 variants. Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2500276122. doi: 10.1073/pnas.2500276122. Epub 2025 Jul 9.
Contact
Toni Meister
07/22/2025
NUM Specialist Network for Infections has been launched
The Fachnetzwerk Infektionen within the Netzwerks Universitätsmedizin (NUM) is now operational.
The first patient with Dengue fever has provided their biological samples and clinical data to the network at the University Medical Center Hamburg-Eppendorf (UKE). This specialist network supports clinical and epidemiological studies in infectious diseases and is the first of its kind within the NUM study network. Its aim is to implement clinical studies more quickly and efficiently. To this end, clinical data and biological samples from patients with infectious diseases are systematically collected at multiple sites. These data are intended to serve as a foundation to advance medical research in Germany, develop new treatment approaches, and enable early, well-informed responses to future pandemics.
A total of 15 university institutions are participating in an initial study of the network. The study leader at UKE is Prof. Dr. Marylyn Addo, Director of the Institute for Infectious Disease Research and Vaccine Development and Head of the Infectiology Section at the Department of Medicine I.
Contact for queries Prof. Dr. Marylyn Addo
06/23/2025
Launch of the European Vaccines Hub for Pandemic Readiness
The European Health Emergency Preparedness and Response Authority (HERA) of the European Commission supports the establishment of the *European Vaccines Hub for Pandemic Readiness (EVH)*, a pan-European center aimed at advancing the development of vaccines for public health.
The University Medical Center Hamburg-Eppendorf (UKE) is also involved in this initiative. The vaccine development efforts will focus on a selected group of pathogens identified as critical for pandemic preparedness in a recent WHO report on pathogen prioritization for the European region.
The EVH consortium comprises a total of 11 beneficiaries and 13 associated and partner institutions from seven countries. It is coordinated by the non-profit organization *Sclavo Vaccines Association*. The project will be supported by approximately €102 million from the EU4Health program of the European Union over the next four years. Of this, around €21 million will go to German institutions within the framework of the German Center for Infection Research (DZIF), including UKE.
For further inquiries, please contact Prof. Dr. Marylyn Addo
6/23/2025
IIRVD & CRC1648 goes HafenCity Run
On May 18, 2025, our team “Go Viral” joined the HafenCity Run – Hamburg’s largest corporate charity run. It’s a great event to stay active while supporting various social projects in Hamburg. Our team – a lively group of 30 colleagues from CRC 1648, IIRVD, and friends – truly lived up to the name. Filled with energy, team spirit, and lots of smiles, “Go Viral” took on the 4-kilometer route through Hamburg’s vibrant HafenCity.
5/18/2025
How the Immune System Responds to Vaccines – Marie Weskamm Receives Funding for Early Vaccine Response Study
Why do some people mount strong immune responses after vaccination, while others produce few or no antibodies? Dr. Marie Weskamm, scientist in the IIRVD is investigating this question. To do so she has received €35,000 from the Werner Otto Foundation Hamburg to fund her independent research.
Congratulations from the IIRVD team!
Publication List
M. Weskamms work
5/15/2025
Successful Retreat of the IIRVD in Alt Duvenstedt
The IIRVD retreat on April 23-24, 2025, in Alt Duvenstedt was a great success. The team addressed and worked through a variety of topics effectively. The team activities fostered a positive atmosphere, and there was lots of laughter. Additionally, the event provided a valuable opportunity to get to know the new colleagues in the Hölzemer working group better.
We look back on a productive and enjoyable time.
04/25/2025
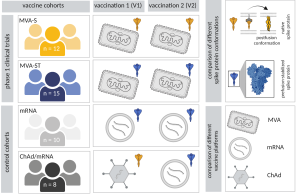
New Publication on the effect of antigen conformation and vaccine platform on early immune responses
The development of vaccines against emerging pathogens can be accelerated by the use of vaccine platforms such as viral vectors or mRNA lipid nanoparticles, as they are based on technologies with known safety profiles and established manufacturing processes. A gene encoding an antigen of an emerging pathogen can be rapidly incorporated into these vaccine platforms.
Different vaccine platforms can induce distinct immune signatures, and innate immune responses induced by viral vector vaccines are suggested to have an adjuvant effect on subsequent adaptive immunity. However, the differential impact of platform and antigen insert on vaccine immunogenicity is not fully understood.
In the present study, we investigated early immune responses to different SARS-CoV-2 vaccines, comparing different vaccine platforms and different conformations of the vaccine antigen. In addition to the approved vaccines ChAdOx1 nCov-19, mRNA-1273 and BNT162b2, we evaluated two vaccine candidates based on the Modified Vaccinia virus Ankara (MVA) vector encoding the native and prefusion-stabilized spike protein, respectively.
Overall, our results show that both the vaccine platform and the antigen insert can influence the early innate immune response and subsequent immunogenicity of a vaccine.
Publication:
Grewe I, Friedrich M, Dieck ML, Spohn M, Ly ML, Krähling V, Mayer L, Mellinghoff SC, Rottstegge M, Kraemer R, Volz A, Becker S, Fathi A, Dahlke C, Weskamm LM, Addo MM. MVA-based SARS-CoV-2 vaccine candidates encoding different spike protein conformations induce distinct early transcriptional responses which may impact subsequent adaptive immunity. Front Immunol. 2024 Dec 19;15:1500615. doi: 10.3389/fimmu.2024.1500615
Contact
Leonie Marie Weskamm
Contact Ilka Grewe
01/10/2025
Leonie Mayer receives doctorate prize
Leonie Mayer has been awarded the Gebhard Koch PhD Prize for Immunology and Virology by the UKE Friends and Patrons for her PhD thesis entitled “Spike-specific T cell immunity following vaccination against the human coronaviruses SARS-CoV-2 and MERS-CoV”.
Congratulations from the IIRVD team!
Publication List
L. Mayer publications
12/13/2024
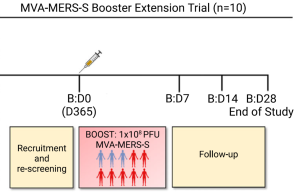
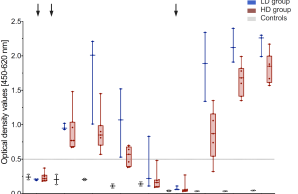
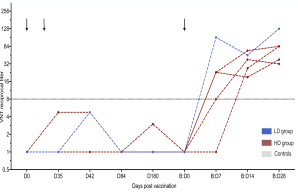
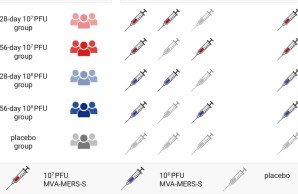
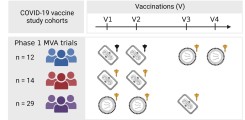
New publication on the safety, immunogenicity, and optimal dosing of a MVA-based vaccine against MERS-CoV
The Middle East Respiratory Syndrome Coronavirus (MERS-CoV) is a WHO priority pathogen of concern that causes severe disease in humans with no licensed vaccine available. MVA-MERS-S is one of the three vaccine candidates currently in early-stage clinical development.
Marylyn Addo and team previously showed in a Phase 1a trial, that MVA-MERS-S is safe and immunogenic in healthy adults ( Koch et al, 2020 ). A second-generation MVA-MERS-S has now been tested in a randomized, placebo-controlled, double-blind Phase clinical 1b trial, to assess the safety, immunogenicity, and optimal dose schedule of the vaccine candidate in humans NCT04119440). The first results of this trial were published by Raadsen, Fathi & Dahlke et. al in the Journal Lancet Infectious Diseases.
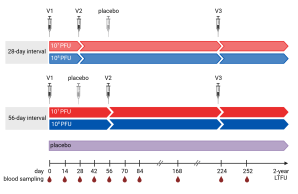
The study was conducted at the University Medical Center Hamburg-Eppendorf and the Erasmus Medical Center in Rotterdam. 140 healthy adults were enrolled and randomized to one of four treatment arms or the placebo arm. Participants of the treatment arms received three vaccinations of either low dose (10⁷ PFU) or high dose (10⁸ PFU) MVA-MERS-S and with either a short (28 days) or a long (56 days) interval between the first and second vaccination.
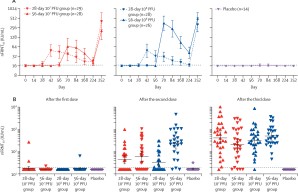
While all tested dose schedules were safe and well tolerated, differences in immunogenicity were observed between the treatment arms. After the second vaccination, neutralizing and S1-specific antibody titers were most robust in the treatment arm that received the high dose of MVA-MERS-S with a prolonged 56-day prime-boost interval. Subsequent third booster vaccination increased antibody responses to comparable levels in all treatment arms.
Detailed safety and immunogenicity results, including vector-specific and cross-reactive antibody responses are described.
While this study describes the first clinical trial to investigate the optimal dose schedule of a MERS vaccine, it also describes the first trial to assess a MERS vaccine in a SARS-CoV-2-exposed population. The published results show, that humoral immunogenicity can be improved by increasing the vaccine dose and prolonging the interval between vaccinations, which may be transferable to other vaccines.
This trial was conducted by an international consortium comprising the Erasmus Medical Center Rotterdam and several institutes of the German Center for Infection Research (DZIF). It was supported by the Coalition for Epidemic Preparedness Innovation (CEPI).
Many thanks to all participants in the MVA-MERS-S study.
Publication:
Raadsen MP, Dahlke C, Fathi A, Hardtke S, Klüver M, Krähling V, Gerresheim GK, Mayer L, Mykytyn AZ, Weskamm LM, Zoran T, van Gorp ECM, Sutter G, Becker S, Haagmans BL, Addo MM; MVA-MERS-S_DF-1 Study group. Safety, immunogenicity, and optimal dosing of a modified vaccinia Ankara-based vaccine against MERS-CoV in healthy adults: a phase 1b, double-blind, randomised placebo-controlled clinical trial. Lancet Infect Dis. 2024 Oct 7:S1473-3099(24)00423-7. doi: 10.1016/S1473-3099(24)00423-7. Epub ahead of print. PMID: 39389076.
Raadsen, Dahlke, Fathi et al, 2024
Contact Leonie Mayer
10/08/2024
Dr. Dr. Ulrike Lange receives NIH grant for pioneering HIV research
A research team led by Dr. Dr. Ulrike Lange, head of the LIV-BMBF junior research group Genomics of Retroviral Infections and associated working group at the IIRVD, has been awarded a five-year NIH (National Institutes of Health) grant of 2.79 million US dollars for the development of an innovative gene therapy that can inactivate the HIV provirus in infected immune cells.
Congratulations to Ulrike and her team for this great success!
Link to the press release
Contact Ulrike Lange
08/27/2024
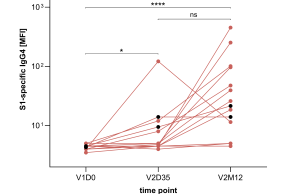
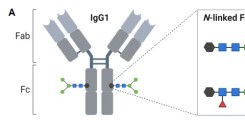
New publication on the induction of noninflammatory SARS-CoV-2 spike-specific IgG4 antibodies after BNT162b2 vaccination in children
IgG antibodies are composed of different subclasses (IgG1-4) with distinct characteristics. While the IgG1 and IgG3 subclasses have a high functional capacity and are typically induced by protein antigens, an atypical induction of IgG2 and IgG4 subclasses has been observed at late time points following mRNA-based vaccination against COVID-19 in adults. We here report similar findings in children aged between five and eleven years, which showed a delayed induction of IgG2 and IgG4 twelve months after a second vaccination with the mRNA vaccine BNT162b2. The immunological mechanisms and clinical implications of these findings remain to be elucidated in future studies.
Publication:
Kobbe R, Rau C, Schulze-Sturm U, Stahl F, Fonseca-Brito L, Diemert A, Lütgehetmann M, Addo MM, Arck P, Weskamm LM. Delayed Induction of Noninflammatory SARS-CoV-2 Spike-Specific IgG4 Antibodies Detected 1 Year After BNT162b2 Vaccination in Children. Pediatr Infect Dis J. 2024
Contact Leonie Marie Weskamm
Contact Robin Kobbe
08/26/2024
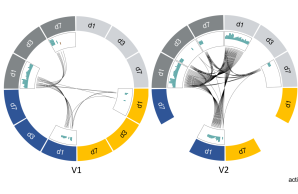
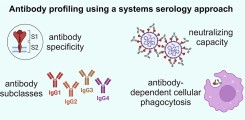
New publication on Fc-mediated antibody functions following vaccination with MVA-MERS-S
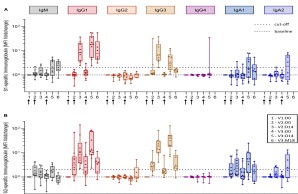
Neutralizing antibodies are considered an important measure for vaccine immunogenicity. In addition, Fc-mediated antibody functions can contribute to antibody-mediated protection. These functions include the recruitment and activation of innate immune cells, and are strongly influenced by structural antibody properties such as subclass and Fc glycan composition. We here applied a systems serology approach to dissect humoral immune responses induced by MVA-MERS-S. MVA-MERS-S is a vaccine against the Middle East respiratory syndrome coronavirus (MERS-CoV) based on the MVA viral vector, which as been evaluated for safety and immunogenicity in previous studies. The results presented in this manuscript show that a late booster vaccination significantly improved both the neutralization capacity and Fc functionality of antibodies, compared to the primary vaccination series. Distinct characteristics were observed for antibodies specific to the MERS-CoV spike protein S1 and S2 subunits, regarding subclass and glycan compositions as well as Fc functionality. These findings highlight the benefit of a late homologous booster vaccination with MVA-MERS-S and may be of interest for the design of future coronavirus vaccines.
Publication:
Leonie M. Weskamm, Paulina Tarnow, Charlotte Harms, Melanie Huchon, Matthijs P. Raadsen, Monika Friedrich, Laura Rübenacker, Cordula Grüttner, Mariana G. Garcia, Till Koch, Stephan Becker, Gerd Sutter, Edouard Lhomme, Bart L. Haagmans, Anahita Fathi, Sandra M. Blois, Christine Dahlke, Laura Richert, Marylyn M. Addo. Dissecting humoral immune responses to an MVA-vectored MERS vaccine in humans using a systems serology approach. iScience. 2024
Contact Marie Weskamm
07/22/2024
New Collaborative Research Centre 1648
As announced by the German Research Foundation (DFG) on May 31st, the UKE will receive a new Collaborative Research Center (CRC) 1648 - "Emerging Viruses: Pathogenesis, Structure, Immunity"
The World Health Organization (WHO) has presented a list of diseases that have the potential to cause future epidemics. These diseases should be prioritized in the development of diagnostics, medications, and vaccines. Common among the diseases on the current WHO Blueprint Priority List, such as Lassa fever, Ebola virus disease, and Middle East Respiratory Syndrome (MERS), is that they are caused by RNA viruses.
The CRC 1648, which will receive approximately 10.8 million euros from the DFG over the next four years, aims to research the fundamental structures and mechanisms of these viral infections. The goal is to improve therapeutic and preventive measures and enable faster responses to outbreaks.
The project involves 26 researchers from the UKE, UHH, Leibniz Institute of Virology (LIV), Bernhard Nocht Institute for Tropical Medicine (BNITM), Friedrich Loeffler Institut (FLI), Hannover Medical School (MHH), Universität zu Lübeck and University Hospital Basel. Together, they will work on understanding infection processes even more precisely at the molecular level. To this end, they will use state-of-the-art techniques from cell and structural biology, computational biology, immunology and biochemistry.
CRCs are long-term, interdisciplinary research collaborations that are funded over a maximum of twelve years and three phases. In this way, the DFG supports excellent and comprehensive collaborative projects in basic research.
Marylyn Addo
, Director of IIRVD, is the speaker of the CRC 1648.
Maya Topf
from LIV/UKE/ CSSB and
Chris Meier
from UHH are the co-speakers.
05/31/2024
Portrait of newcomers
The "SYNERGIE" magazine of the German Centers for Health Research (DZG) is published twice a year and provides information on projects and successes in translational research.
In the current issue, Dr. Toni Luise Meister talks about her career in infection research to date and her work as head of a junior research group funded by the DZIF.
Link
to the article in SYNERGIE magazine (article in German)
Contact T. Meister
05/27/2024
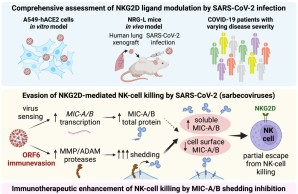
Mechanism for immune evasion of SARS-CoV-2 identified
An international research team, including Angelique Hölzemer, associate group leader at the IIRVD, has demonstrated how SARS-CoV-2 and its variants evade the human immune system. The results of the study indicate that cells infected with SARS-CoV-2 downregulate important immune signals, namely NKG2D ligands. These ligands play a pivotal role in activating the immune system, particularly natural killer cells (NK cells), in order to combat viral infections.
These findings could contribute to the development of new therapeutic approaches against Covid-19, particularly given that the study also demonstrates the importance of NK cells in combating SARS-CoV-2-infected cells.
Hartmann JA, Cardoso MR, Talarico MCR, Kenney DJ, Leone MR, Reese DC, Turcinovic J, O'Connell AK, Gertje HP, Marino C, Ojeda PE, De Paula EV, Orsi FA, Velloso LA, Cafiero TR, Connor JH, Ploss A, Hoelzemer A, Carrington M, Barczak AK, Crossland NA, Douam F, Boucau J, Garcia-Beltran WF. Evasion of NKG2D-mediated cytotoxic immunity by sarbecoviruses. Cell. 2024 May 9;187(10):2393-2410.e14. doi: 10.1016/j.cell.2024.03.026. Epub 2024 Apr 22. PMID: 38653235 Hartmann et al
Angelique Hölzemer is a specialist at the I. Medical Clinic and Polyclinic of the UKE and head of the Infection & Immune Regulation working group at the IIRVD and the Leibniz Institute of Virology (LIV).
05/09/2024
Ehren-Schleusenwärter at BNITM
In her role as 'Ehren-Alster-Schleusenwärterin', Marylyn Addo invited other 'Ehren-Alster-Schleusenwärter and 'Ehren-Alster-Schleusenwärterinnen' to the Bernhard Nocht Institute for Tropical Medicine (BNITM) to introduce her field of work. Prof. Jürgen May, Director of the BNITM, began with a presentation on the history of the BNITM.
The 'Congregation der Alster-Schleusenwärter S.C.' appoints one or more 'Ehren-Schleusenwärter' each year. This award is given to individuals who have enhanced the reputation of the Free and Hanseatic City of Hamburg in the world through special achievements.
04/24/2024
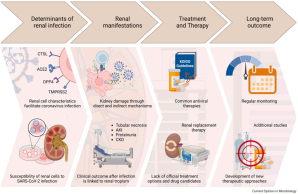
New Review
Valentin Bärreiter and Toni Meister have published a new review on renal implications of coronavirus disease in the journal Current Opinion in Microbiology.
Click here to read the Review
04/24/2024
Congratulations, Dr. Mayer!
Leonie Mayer has very successfully defended her PhD thesis entitled: "Spike-specific T cell immunity following vaccination against the human coronaviruses SARS-CoV-2 and MERS-CoV".
The IIRVD sincerely congratulates her on her success!
Publication List
L. Mayer publications
04/09/2024
New Publication on the immunogenicity of MVA-based vaccine candidates against COVID-19
In response to the COVID-19 pandemic, multiple vaccines were developed using platforms such as viral vectors and mRNA technology. Here, we report humoral and cellular immunogenicity data from human phase 1 clinical trials investigating two SARS-CoV-2 vaccine candidates: Based on the recombinant viral vector Modified Vaccinia virus Ankara (rMVA), MVA-SARS-2-S (link to NCT04569383) and MVA-SARS-2-ST (link to NCT04895449) encode the native and the prefusion-stabilized SARS-CoV-2 spike protein, respectively. MVA-SARS-2-ST was more immunogenic than MVA-SARS-2-S, but both were less immunogenic compared to licensed mRNA- and ChAd-based vaccines in SARS-CoV-2 naïve individuals. In heterologous vaccination, previous MVA-SARS-2-S vaccination enhanced T cell functionality and MVA-SARS-2-ST boosted the frequency of T cells and S1-specific IgG levels when used as a third vaccination. While the vaccine candidate containing the prefusion-stabilized spike elicited predominantly S1-specific humoral and T cell responses, immunity to the candidate with the native spike was skewed towards S2-specific responses. These data demonstrate how the spike antigen conformation, using the same viral vector, directly affects vaccine immunogenicity in humans.
Leonie Mayer, Leonie M. Weskamm, Anahita Fathi, Maya Kono, Jasmin Heidepriem, Verena Krähling, Sibylle C. Mellinghoff, My Linh Ly, Monika Friedrich, Svenja Hardtke, Saskia Borregaard, Thomas Hesterkamp, Felix F. Loeffler, Asisa Volz, Gerd Sutter, Stephan Becker, Christine Dahlke, Marylyn M. Addo. MVA-based vaccine candidates encoding the native or prefusion-stabilized SARS-CoV-2 spike reveal differential immunogenicity in humans. npj Vaccines 2024 Jan 26;9(1):20. doi: 10.1038/s41541-023-00801-z
Contact Leonie Mayer
01/30/2024
New Publication on the CD8+ epitope response following MVA-MERS-S vaccination
Harrer & Mayer et al. used an epitope-mapping approach to identify for the first time in humans a MERS-CoV-spike-specific CD8+ T-cell epitope (amino acid sequence: GLFPYQGDHGDMYVY), which is likely HLA-B*35:01 restricted.
Licensed vaccines against the Middle East respiratory syndrome coronavirus (MERS-CoV), an emerging pathogen of concern, are lacking. The Modified Vaccinia virus Ankara vector-based vaccine MVA-MERS-S, expressing the MERS-CoV-spike glycoprotein (MERS-S), is one of three candidate vaccines in clinical development and elicits robust humoral and cellular immunity ( link to MERS 1a trial ). Here, we identified for the first time a MERS-S-specific CD8+ T-cell epitope in an HLA-A*03:01/HLA-B*35:01-positive vaccinee using a screening assay, intracellular cytokine staining, and in silico epitope prediction. As evidence from MERS-CoV infection suggests a protective role of long-lasting CD8+ T-cell responses, the identification of epitopes will facilitate longitudinal analyses of vaccine-induced T-cell immunity.
Caroline E. Harrer, Leonie Mayer, Anahita Fathi, Susan Lassen, My L. Ly, Madeleine E. Zinser, MVA-MERS-S Study Group, Timo Wolf, Stephan Becker, Gerd Sutter, Christine Dahlke, Marylyn M. Addo. Identification of a spike-specific CD8+ T cell epitope following vaccination against the Middle East respiratory syndrome coronavirus in humans. The Journal of Infectious Diseases 2024 Jan 19; jiad612 [online ahead of print]. doi: 10.1093/infdis/jiad612
This project is a result of an MD stipend funded by the German Center for Infection Research (DZIF).
Contact Leonie Mayer
01/19/2024
Marylyn Addo has been elected as a new member of the Academy of Sciences in Hamburg
The eight new members were officially welcomed at the Academy's annual public celebration in Hamburg on November 17, 2023 ( Link ). The picture shows Prof. Dr. Mojib Latif, President of the Academy, handing over the membership certificate to Marylyn Addo.
The Hamburg Parliament founded the Academy of Sciences in Hamburg in 2004 in order to intensify research across disciplinary and institutional boundaries and to increase the visibility of the scientific region of northern Germany. Renowned scientists from northern Germany are involved in the academy. The academy was conceived as a workplace. Its members develop interdisciplinary research topics in working groups and project groups. These projects deal with important social issues and fundamental scientific problems. In addition, the Academy promotes discussion between science and the public on these topics.
11/18/2023
The IIRVD welcomes its new members
In October, the IIRVD is pleased to welcome four new scientists.
Valentin Bärreiter is embarking on his PhD thesis, while Toni Louise Meister joins as Senior Postdoc with the DZIF-funded position in the research area of "Emerging Infections".
Mascha Binder from the Universitätsspital Basel, Switzerland and Christoph Lange from the Research Center Borstel are visiting scholars who will work closely with the IIRVD in the future.
The IIRVD extends a warm welcome to the new staff members and values their highly qualified support.
10/10/2023
Marylyn Addo receives Federal Cross of Merit
Prof. Addo was awarded the Federal Cross of Merit with Ribbon for her outstanding medical and scientific achievements in the research of infectious diseases such as HIV, Ebola and Covid-19 as well as for her dedicated social commitment.
Marylyn Addo received the award from Science Senator Katharina Fegebank on August 25, 2023 in recognition of her achievements. In her speech, Prof. Addo explained that she was accepting the order on behalf of all those who made important contributions to care and medical services during the pandemic, as well as now, and for scientists in a variety of disciplines.
08/23/2023
IIRVD Summer Team Event
In summery temperatures the IIRVD spent a great afternoon in the Kletterwald Hamburg.
Everyone mastered their personal challenge and had their own personal sense of achievement.
It was a lot of fun for everyone, whether on the ground or in the trees.
07/11/2023
Congratulations, Dr. Weskamm!
Leonie Marie Weskamm has successfully defended her doctoral thesis entitled: "Characterization of B cell and antibody responses induced by vaccination against the human coronaviruses MERS-CoV and SARS-CoV-2".
We sincerely congratulate her on her success.
Publication List
L. M. Weskamm
07/07/2023
Anahita Fathi wins the Meta-Alexander Prize for Clinical Infection Research
This year's 2023 Meta-Alexander Prize for Clinical Infection Research was awarded to Dr. Anahita Fathi for her work "Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrom" published in Nature Communications.
This science prize of the German Society of Infectious Diseases ( DGI ) Prize was awarded during the 16th Congress of Infectious Diseases and Tropical Medicine in Leipzig.
Original publication:
Fathi, A., Dahlke, C., Krähling, V. et al. Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome. Nat Commun 13, 4182 (2022).
Open access
This year, the Meta-Alexander-Prize was split between two winners.
Picture: Photographer Tom Dachs/ Copyright: @Tom Dachs/KIT2023
06/26/2023
HafenCity Run 2023
On June 3, 2023, the motivated team "GO VIRAL" of the IIRVD participated in the HafenCity Run. The HafenCity Run combines sports, team spirit and charity in one event.
With its participation, the IIRVD supports the charitable projects of the charity partner Hamburger Abendblatt hilft e.V..
06/03/2023
Diversity-Tag 2023
The IIRVD also wants to set a sign for more diversity. By wearing different socks, we jointly set a sign for diversity in society and in the working world.
05/23/2023
World Immunization Week 2023
Interview with Prof. Dr. Addo on protection through vaccination
On the occasion of the World Immunization Week 2023 initiated by the WHO, Prof. Dr. Marylyn Addo explains which infectious diseases children and adults should be vaccinated against, whether vaccinations missed due to the pandemic can be catched up for and which vaccines her institute is currently investigating. (Video in German)
Leonie Mayer on her motivation to research vaccines.
Leonie Mayer, a doctoral student in natural sciences at the IIRVD, talks about her research at the institute in an Instagram post.Her doctoral research involves examining samples from the institute's clinical trials to find out how immune responses follow vaccinations. With her research findings, she hopes to help improve vaccines and help as many people as possible.
Link to videos.
04/28/2023
New publication on flow cytometric characterization of memory B cells directed against SARS-CoV-2 spike proteins.
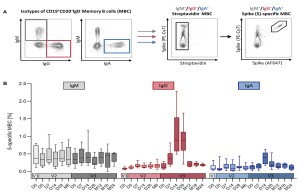
Memory B cells (MBCs), part of the immune response elicited by infection or vaccination, can persist in lymphoid organs and peripheral blood and are capable of rapid reactivation upon secondary antigen exposure. Here, we describe a flow cytometric assay to identify SARS-CoV-2 spike-specific MBCs from peripheral blood mononuclear cells and characterize their isotypes and activation status.
Leonie M. Weskamm, Christine Dahlke, Marylyn M. Addo. Flow cytometric protocol to characterize human memory B cells directed against SARS-CoV-2 spike protein antigens. STAR Protocols 2022 Dec 16; 4(3):101902. doi: 10.1016/j.xpro.2022.101902
Contact Leonie Marie Weskamm
12/15/2022
Why does a small injection helps against infectious diseases?
On November 14, 2022, Prof. Addo gave a presentation at the "Kinder-Uni Hamburg" ( Link ).
In her lecture, Prof. Addo explained to the young audience why certain infectious diseases can spread quickly around the world, how these diseases are treated and how vaccines can help. She also gave tips on what everyone can do to protect themselves from viruses.
Further information on the Kinder-Uni Hamburg can be found
here
.
12/12/2022
New publication on the B and T cell responses elicited by the MVA-MERS-S vaccine candidate
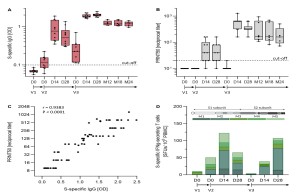
Weskamm et al. longitudinally describe B and T cell responses as well as antibody subclasses and neutralization capacity induced by three homologous immunizations with the MVA-MERS-S vaccine candidate.
The Middle East Respiratory Syndrome (MERS) is a respiratory disease caused by MERS coronavirus (MERS-CoV). In follow-up to a phase 1 trial , we perform a longitudinal analysis of immune responses following immunization with the Modified Vaccinia virus Ankara (MVA)-based vaccine MVA-MERS-S encoding the MERS-CoV-spike protein. Three homologous immunizations were administered on days 0 and 28 with a late booster vaccination at 12±4 months. Antibody isotypes, subclasses and neutralization capacity as well as T and B cell responses were monitored over a period of three years using standard and bead-based ELISA, PRNT50, ELISpot and flowcytometry. The late booster immunization significantly increases frequency and persistence of spike-specific B cells, binding IgG1 and neutralizing antibodies, but not T cell responses. Our data highlight the potential of a late boost to enhance long-term antibody and B cell immunity against MERS-CoV. Our findings on the MVA-MERS-S vaccine may be of relevance for COVID-19 vaccination strategies.
Weskamm LM, Fathi A, Raadsen MP, Mykytyn AZ, Koch T, Spohn M, Friedrich M; MVA-MERS-S Study Group, Haagmans BL, Becker S, Sutter G, Dahlke C, Addo MM. Persistence of MERS-CoV-spike-specific B cells and antibodies after late third immunization with the MVA-MERS-S vaccine. Cell Rep Med. 2022 Jul 19;3(7):100685. doi: 10.1016/j.xcrm.2022.100685. Weskamm et al.
Contact Leonie Marie Weskamm
07/21/2022
New publication on the MVA-MERS-S booster vaccination in Nature Communications
Fathi et al. evaluate the safety and immunogenicity of a third vaccination with MVA-MERS-S in a subgroup of trial participants one year after primary immunization Link to vaccine trial . The results show that MVA-MERS-S booster vaccination is safe and well-tolerated. Both binding and neutralizing anti-MERS-CoV antibody titers increase substantially in all participants and exceed maximum titers observed after primary immunization more than 10-fold.
The data by Fathi et al. support the rationale of a booster vaccination with MVA-MERS-S and encourage further investigation in larger trials.
Fathi A, Dahlke C, Krähling V, Kupke A, Okba NMA, Raadsen MP, Heidepriem J, Müller MA, Paris G, Lassen S, Klüver M, Volz A, Koch T, Ly ML, Friedrich M, Fux R, Tscherne A, Kalodimou G, Schmiedel S, Corman VM, Hesterkamp T, Drosten C, Loeffler FF, Haagmans BL, Sutter G, Becker S, Addo MM. Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome. Nat Commun. 2022 Jul 19;13(1):4182. doi: 10.1038/s41467-022-31557-0 Fathi et al.
07/20/2022
Prof. Dr. Addo honored for HIV research
Prof. Dr. Marylyn Addo, Director of the Institute for Infection Research and Vaccine Development, has been awarded the Heinz Ansmann Prize for AIDS Research 2022. The Heinz Ansmann foundation honored her work on immune control of HIV infection and transfer of knowledge to other viral diseases with pandemic potential. Prof. Dr. Addo accepted the 10,000 euro prize on June 13, 2022 at Schloss Mickeln in Düsseldorf. Prof. Florian Klein from Cologne was awarded the 2020 Price the same day.
06/13/2022


























_contentbild_hochformat.jpg)


_contentbild.jpg)