Radiobiology of Pediatric Brain Tumors
Dr. rer. nat. Nina Struve (Biochemist, Medical Scientist)
PD Dr. med. Martin Mynarek(Pediatric Hematology & Oncology, Pediatric oncologist, Clinician Scientist)
Britta Riepen (MTA)
Alexander Schramm (BTA)
Nele Köppen (Biology MSc Student)
Marie Hintelmann (Biotechnology student)
Mirja Gerbach (PhD student)
Joel Senador (PhD student)
Hans Halben (Medical student)
Henrik Gampert (Physics student)
Topic
Medulloblastoma
Medulloblastoma (MB) is the most frequent high-grade (WHO IV) brain tumor of childhood and adolescence. Metastasis is one of the key clinical problems in MB. The multimodal therapy of MB consists of surgery, chemotherapy and radiotherapy with the latter being considered the most effective modality. However, radiotherapy causes a magnitude of relevant late-effects in survivors. Most importantly, it interferes with neurocognitive development especially in younger children.
MB itself is a heterogeneous disease with at least four different biological groups according to the 2021 WHO classification of tumors of the CNS: WNT, SHH-TP53mutant, SHH-TP53 wildtype and non-WNT/non-SHH. With the exception of (rare) early childhood SHH-TP53 wildtype MB, radiation-sparing regimens are associated with poor outcomes. However, even after maximally dose-escalated CSI together with intensive combination chemotherapy, metastatic non-WNT/non-SHH MB has progression-free survival (PFS) rates of only 60%.
Despite the high importance of radiotherapy for disease control, the mechanisms underlying response and resistance to radiotherapy are incompletely understood. Our aim is to characterize the intrinsic cellular radiosensitivity, DNA repair pathways and capacities present in the different MB subgroups. Thereby, we will focus on DNA double-strand break (DSB) repair (in vitro and ex vivo analysis), which is known primarily to determine the cellular radiosensitivity of the individual tumor. Furthermore, we are investigating new treatment options for MB with a special focus on radiosensitizing approaches. The group has a very close link to the pediatric brain tumor clinical research unit at the Department for Pediatric Hematology and Oncology, which provides access to relevant clinical data and potentially confirmation of preclinical results in clinical trials.
Following projects are ongoing:
NeuStaRT
The joint BMBF-funded project "NeuStaRT" (in collaboration with the University Hospital Essen) aims to develop new targeted and innovative multimodal (radio-)therapy concepts to improve the prognosis of MB patients. Thereby we will focus not only on the efficacy of combined treatments, but also on the impact of certain treatments on neurotoxicity.
BEETLE
In our subproject, which is part of the BMBF-funded project “BEETLE” (in collaboration with DESY, TRUMPF Scientific Lasers GmbH, Active Fiber Systems GmbH) we are currently investigating the effect of ultra-high dose rate radiotherapy called FLASH RT on MB and normal human cells. In the last years, much attention has been drawn to the implementation of ultra-high dose rate radiotherapy into the clinic. This irradiation method, which is usually characterized by a mean dose rate > 40 Gy/s has been proven to better spare normal tissue than conventional delivery (characterized by a mean dose rate of about 0.1 Gy/s), without impacting tumor control.
MSNZ Partner Lab
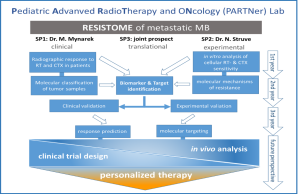
This project is supported by the Mildred-Scheel Cancer Career Center HATRICS4 with Dr. Struve and PD Dr. Mynarek as the leading scientists. Project title: The RESISTOME of metastatic medulloblastoma: Unravelling molecular mechanisms for personalized therapy.
Fig. 1: Overview of the MSNZ partner lab projects addressing relevant research questions regarding the radiobiology of MB a on a pre-clinical and clinical level.
Collaborations
Within the UCCH/MNSZ there is a strong collaboration especially with the Clinic for radiotherapy, the Neuropathology, the Center for Molecular Neurology (ZMNH), the Children´s Cancer center Hamburg, the Pediatric Hematology & Oncology and also with external departments at the DKFZ, University of Essen, Tübingen, Düsseldorf, Münster, Berlin etc.
Funding
BMBF, Mildred Scheel Cancer Center (German Cancer Aid), Brigitte und Dr. Konstanze Wegener-Stiftung,
Selected Publications:
Tonn S, Korshunov A, Obrecht D, Sill M, Spohn M, von Hoff K, Milde T, Pietsch T, Goschzik T, Bison B, Juhnke BO, Struve N, Sturm D, Sahm F, Bockmayr M, Friedrich C, von Bueren AO, Gerber NU, Benesch M, Jones DTW, Kool M, Wefers AK, Schüller U, Pfister SM, Rutkowski S, Mynarek M. Risk prediction in early childhood sonic hedgehog medulloblastoma treated with radiation-avoiding chemotherapy: Evidence for more than 2 subgroups. Neuro Oncol. 2023 Aug 3;25(8):1518-1529. doi: 10.1093/neuonc/noad027. PMID: 36715306; PMCID: PMC10398808.
Goschzik T, Mynarek M, Doerner E, Schenk A, Spier I, Warmuth-Metz M, Bison B, Obrecht D, Struve N, Kortmann RD, Schmid M, Aretz S, Rutkowski S, Pietsch T. Genetic alterations of TP53 and OTX2 indicate increased risk of relapse in WNT medulloblastomas. Acta Neuropathol. 2022 Oct 1. doi: 10.1007/s00401-022-02505-5. Epub ahead of print. PMID: 36181537.
Korshunov A, Okonechnikov K, Stichel D, Schrimpf D, Delaidelli A, Tonn S, Mynarek M, Sievers P, Sahm F, Jones DTW, von Deimling A, Pfister SM, Kool M. Gene expression profiling of Group 3 medulloblastomas defines a clinically tractable stratification based on KIRREL2 expression. Acta Neuropathol. 2022 Aug;144(2):339-352. doi: 10.1007/s00401-022-02460-1. Epub 2022 Jun 30. PMID: 35771282; PMCID: PMC9288368.
Obrecht D, Mynarek M, Hagel C, Kwiecien R, Spohn M, Bockmayr M, Bison B, Pfister SM, Jones DTW, Sturm D, von Deimling A, Sahm F, von Hoff K, Juhnke BO, Benesch M, Gerber NU, Friedrich C, von Bueren AO, Kortmann RD, Schwarz R, Pietsch T, Fleischhack G, Schüller U, Rutkowski S. Clinical and molecular characterization of isolated M1 disease in pediatric medulloblastoma: experience from the German HIT-MED studies. J Neurooncol. 2022 Mar;157(1):37-48. doi: 10.1007/s11060-021-03913-5. Epub 2022 Feb 21. PMID: 35190934; PMCID: PMC8938370.
Struve N, Hoffer K, Weik AS, Riepen B, Krug L, Cetin MH, Burmester J, Ott L, Liebing J, Gatzemeier F, Müller-Goebel J, Gerbach M, Bußmann L, Parplys AC, Unger K, Mansour WY, Schüller U, Rieckmann T, Petersen C, Rothkamm K, Short SC, Kriegs M. Increased replication stress and R-loop accumulation in EGFRvIII-expressing glioblastoma present new therapeutic opportunities. Neurooncol Adv. 2021 Dec 4;4(1):vdab180. doi: 10.1093/noajnl/vdab180. PMID: 35274102; PMCID: PMC8903237.
Struve N, Binder ZA, Stead LF, Brend T, Bagley SJ, Faulkner C, Ott L, Müller-Goebel J, Weik AS, Hoffer K, Krug L, Rieckmann T, Bußmann L, Henze M, Morrissette JJD, Kurian KM, Schüller U, Petersen C, Rothkamm K, O Rourke DM, Short SC, Kriegs M. EGFRvIII upregulates DNA mismatch repair resulting in increased temozolomide sensitivity of MGMT promoter methylated glioblastoma. Oncogene. 2020 Apr;39(15):3041-3055. doi: 10.1038/s41388-020-1208-5. Epub 2020 Feb 17. PMID: 32066879; PMCID: PMC7142016.