Research group "Skeletal Pathobiochemistry"
Head:
Prof. Dr. rer. nat. Sandra Pohl
Heisenberg-Professorship for Subcellular Osteology
Phone:
Mail:
s.pohl(at)uke.de
- Research
- Team
- Publications
- Funding
- Open Positions
-
Research
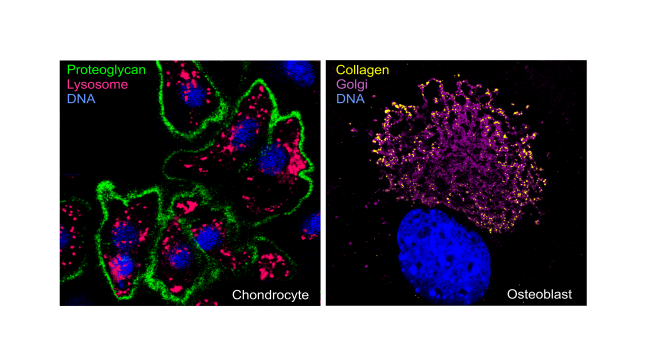
 Proteoglycan staining of chondrocytes
Proteoglycan staining of chondrocytesThe organic skeletal matrix is composed of structurally diverse macromolecules such as collagens, proteoglycans and glycoproteins. These components are synthesized and posttranslationally modified in the endoplasmic reticulum and the Golgi apparatus of chondrocytes in the cartilage and osteoblasts in the bone tissue, and finally secreted. On the other hand, degradation of skeletal matrix macromolecules is mediated by hydrolytic enzymes such as lysosomal enzymes of the matrix-resident cells. The development and maintenance of the skeleton requires constant turnover of the highly dynamic skeletal matrix in both bone and cartilage tissues.
Pathophysiology and molecular mechanisms of children’s skeletal disorders
Impaired metabolisms in the bone and cartilage tissue result in a number of disorders specifically in childhood including rare genetically inherited lysosomal storage diseases and osteogenesis imperfecta. Biochemically, these diseases are characterized by deficiency or inactivity of specific proteins that coordinate a plethora of molecular pathways in the bone and cartilage. In turn this results in heterogeneity of skeletal manifestations in the affected patients.Our research group aims to understand the molecular mechanisms balancing skeletal matrix synthesis and degradation and to elucidate the pathophysiological basis of genetically inherited skeletal disorders in childhood. Pursuing these goals, we study mouse and cell-based models of the diseases as well as patient material by the use of combined methods from biochemistry, molecular and cell biology as well as histology and immunhistochemistry in tight collaboration with various research groups both within and beyond IOBM.
-
-
Team
 Prof. Dr. rer. nat.Sandra Pohl
Prof. Dr. rer. nat.Sandra Pohl- Head of research group
Location
Campus Forschung I - N27 , 1st Floor, Room number 01.006 Maike BuckM. Sc.
Maike BuckM. Sc.- Doctoral student
Location
Campus Forschung I - N27 , Ground Floor, Room number 0.007 Kenneth SchöneckM. Sc.
Kenneth SchöneckM. Sc.- Doctoral student
Location
Campus Forschung I - N27 , Ground Floor, Room number 0.007 Funda Celik
Funda Celik- Biological-technical assistant
Location
Campus Forschung I - N27 , 1st Floor, Room number 01.008 Thomas Höger
Thomas Höger- Biological-technical assistant
Location
Campus Forschung I - N27 , 1st Floor, Room number 01.008.1 Location
Location
Campus Forschung I - N27 , 1st Floor Location
Location
Campus Forschung I - N27 , 1st Floor
-
Publications
2025
Absolute quantification of lysosomal proteins by multiple reaction monitoring mass spectrometry and QconCAT protein standards.
Mosen PR, Moharana B, Fajardo-Callejon S, Kaade E, Rösel N, Omidi M, Sakson R, Ruppert T, Pohl S, Gieselmann V, Winter D (2025) bioRxiv https://doi.org/10.1101/2025.01.09.632238 (preprint)2024
Inactivation of spermine synthase in mice causes osteopenia due to reduced osteoblast activity.
Yorgan TA, Zhu Y, Wiedemann P, Schöneck K, Pohl S, Schweizer M, Amling M, Barvencik F, Oheim R, Schinke T (2024) J Bone Miner Res 56. doi: 10.1093/jbmr/zjae156O-GlcNAcylation in the osteoblast lineage - boosting the complexity of Wnt-stimulated bone formation.
Pohl S, Schinke T (2024) EMBO Rep doi: 10.1038/s44319-024-00242-22023
Mice heterozygous for an osteogenesis imperfecta-linked MBTPS2 variant display a compromised subchondral osteocyte lacunocanalicular network associated with abnormal articular cartilage.
Danyukova T, Alimy AR, Velho RV, Yorgan TA, Di Lorenzo G, von Kroge S, Tidow H, Wiegert JS, Hermans-Borgmeyer I, Schinke T, Rolvien T, Pohl S (2023) Bone 177: 116927CNS manifestations in mucolipidosis type II - A retrospective analysis of longitudinal data on neurocognitive development and neuroimaging in eleven patients.
Ammer LS, Täuber K, Perez A, Dohrmann T, Denecke J, Santer R, Blümlein U, Ozga AK, Pohl S, Muschol NM (2023) J Clin Med 18: 4114Non-functional ubiquitin C-terminal hydrolase L1 drives podocyte injury through impairing proteasomes in autoimmune glomerulonephritis.
Reichelt J, Sachs W, Frömbling S, Fehlert J, Studencka-Turski M, Betz A, Loreth D, Blume L, Witt S, Pohl S, Brand J, Czesla M, Knop J, Florea BI, Zielinski S, Sachs M, Hoxha E, Hermans-Borgmeyer I, Zahner G, Wiech T, Krüger E, Meyer-Schwesinger C (2023) Nat Commun 14: 21142022
A homozygous hypomorphic BNIP1 variant causes an increase in autophagosomes and reduced autophagic flux and results in a spondylo-epiphyseal dysplasia.
Holling T, Bhavani GS, von Elsner L, Shah H, Kausthubham N, Bhattacharyya SS, Shukla A, Mortier GR, Schinke T, Danyukova T, Pohl S, Kutsche K, Girisha KM (2022) Hum Mutat. doi: 10.1002/humu.24368Site-1 and site-2 proteases: A team of two in regulated proteolysis.
Danyukova T, Schöneck K, Pohl S. Biochim Biophys Acta Mol Cell Res (2022) 1869: 1191382021
Pathogenic variants in GNPTAB and GNPTG encoding distinct subunits of GlcNAc-1-phosphotransferase differentially impact bone resorption in patients with mucolipidosis type II and III
Di Lorenzo G, Westermann LM, Yorgan TA, Stürznickel J, Ludwig NF, Ammer LS, Baranowsky A, Ahmadi S, Pourbarkhordariesfandabadi E, Breyer SR, Board TN, Foster A, Mercer J, Tylee K, Velho RV, Schweizer M, Renné T, Braulke T, Randon DN, Sperb-Ludwig F, de Camargo Pinto LL, Moreno CA, Cavalcanti DP, Amling M, Kutsche K, Winter D, Muschol NM, Schwartz IVD, Rolvien T, Danyukova T, Schinke T, Pohl S (2021) Genet Med 23: 2369-2377Mucolipidosis type II and type III: a systematic review of 843 published cases
Dogterom EJ, Wagenmakers MAEM, Wilke M, Demirdas S, Muschol NM, Pohl S, Meijden JCV, Rizopoulos D, Ploeg ATV, Oussoren E (2021) Genet Med 23: 2047-2056Transgenic inhibition of interleukin-6 trans-signaling does not prevent skeletal pathologies in mucolipidosis type II mice
Westermann LM, Baranowsky A, Di Lorenzo G, Danyukova T, Soul J, Schwartz JM, Hendrickx G, Amling M, Rose-John S, Garbers C, Schinke T, Pohl S (2021) Sci Rep 11: 3556Is hematopoietic stem cell transplantation a therapeutic option for mucolipidosis type II?
Ammer LS, Pohl S, Breyer SR, Aries C, Denecke J, Perez A, Petzoldt M, Schrum J, Müller I, Muschol NM (2021) Mol Genet Metab Rep 26: 1007042020
Imbalanced cellular metabolism compromises cartilage homeostasis and joint function in a mouse model of mucolipidosis type III gamma
Westermann LM, Fleischhauer L, Vogel J, Jenei-Lanzl Z, Ludwig NF, Schau L, Morellini F, Baranowsky A, Yorgan TA, Di Lorenzo G, Schweizer M, de Souza Pinheiro B, Guarany NR, Sperb-Ludwig F, Visioli F, Oliveira Silva T, Soul J, Hendrickx G, Wiegert JS, Schwartz IVD, Clausen-Schaumann H, Zaucke F, Schinke T, Pohl S*, Danyukova T* (2020) Dis Model Mech 31: 1796-1814Distinct modes of balancing glomerular cell proteostasis in mucolipidosis type II and III prevent proteinuria
Sachs W, Sachs M, Krüger E, Zielinsky S, Kretz O, Huber TB, Baranowsky A, Westermann LM, Velho RV, Ludwig NF, Yorgan TA, Di Lorenzo G, Kollmann K, Braulke T, Schwartz IV, Schinke T, Danyukova T, Pohl S*, Meyer-Schwesinger C* (2020) J Am Soc Nephrol 31: 1796-1814Hip morphology in mucolipidosis type II
Ammer LS, Oussoren E, Muschol NM, Pohl S, Rubio-Gozalbo ME, Santer R, Stuecker R, Vettorazzi E, Breyer SR (2020) J Clin Med 9: E728Enzyme replacement therapy in mice lacking arylsulfatase B targets bone-remodeling cells, but not chondrocytes
Hendrickx G, Danyukova T, Baranowsky A, Rolvien T, Angermann A, Schweizer M, Keller J, Schröder J, Meyer-Schwesinger C, Muschol N, Paganini C, Rossi A, Amling M, Pohl S*, Schinke T* (2020) Hum Mol Genet 29: 803-816Combined in-vitro and in-silico analyses of missense mutations in GNPTAB provide new insights into the molecular bases of mucolipidosis II and III alpha/beta
Danyukova T, Ludwig NF, Velho RV, Harms FL, Güneş N, Tidow H, Schwartz IV, Tüysüz B, Pohl S (2020) Hum Mutat 41: 133-1392019
Toward engineering the mannose 6-phosphate elaboration pathway in plants for enzyme replacement therapy of lysosomal storage disorders
Zeng Y, He X, Danyukova T, Pohl S, Kermode AR (2019)J Clin Med
8: E2190Mice deficient in the lysosomal enzyme palmitoyl-protein thioesterase 1 (PPT1) display a complex retinal phenotype
Atiskova Y, Bartsch S, Danyukova T, Becker E, Hagel C, Storch S, Bartsch U (2019) Sci Rep 9: 14185The lysosomal storage disorders mucolipidosis type II, type III alpha/beta and type III gamma: Update on GNPTAB and GNPTG mutations
Velho RV, Harms FL, Danyukova T, Ludwig NF, Friez MJ, Cathey SS, Filocamo M, Tappino B, Güneş N, Tüysüz B, Tylee KL, Brammeier KL, Heptinstall L, Oussoren E, van der Ploeg AT, Petersen C, Alves S, Saavedra GD, Schwartz IV, Muschol N, Kutsche K, Pohl S (2019) Hum Mutat 40: 842-864Humoral immune response in adult Brazilian patients with mucolipidosis III gamma
Sperb-Ludwig F, Alegra T, Velho RV, Ludwig N, Siebert M, Jobim M, Vairo F, Schwartz IVD (2019) Genet Mol Biol 42: 571-573A newly generated neuronal cell model of CLN7 disease reveals aberrant lysosome motility and impaired cell survival
von Kleist L, Ariunbat K, Braren I, Stauber T, Storch S, DanyukovaT (2019) Mol Genet Metab 126: 196-2052018
The lysosomal protein arylsulfatase B is a key enzyme involved in skeletal turnover
Pohl S, Angermann A, Jeschke A, Hendrickx G, Yorgan TA, Makrypidi-Fraune G, Steigert A, Kühn SC, Rolvien T, Schweizer M, Köhne T, Neven M, Winter O, Velho RV, Albers J, Streichert T, Pestka JM, Baldauf C, Breyer S, Stuecker R, Muschol N, Cox TM, Saftig P, Paganini C, Rossi A, Amling M, Braulke T, Schinke T (2018) J Bone Miner Res 33: 2186-2201Lysosomal proteome and secretome analysis identifies missorted enzymes and their non-degraded substrates in mucolipidosis III mouse cells
Di Lorenzo G, Velho RV, Winter D, Thelen M, Ahmadi S, Schweizer M, De Pace R, Cornils K, Yorgan TA, Grüb S, Hermans-Borgmeyer I, Schinke T, Müller-Loennies S, Braulke T, Pohl S (2018) Mol Cell Proteomics 17: 1612-1626Loss of CLN7 results in depletion of soluble lysosomal proteins and impaired mTOR reactivation
Danyukova T, Ariunbat K, Thelen M, Brocke-Ahmadinejad N, Mole SE, Storch S (2018) Hum Mol Genet 27: 1711-17222017
GNPTAB missense mutations cause loss of GlcNAc-1-phosphotransferase activity in mucolipidosis type II through distinct mechanisms
Ludwig NF, Velho RV, Sperb-Ludwig F, Acosta AX, Ribeiro EM, Kim CA, Gandelman Horovitz DD, Boy R, Rodovalho-Doriqui MJ, Lourenço CM, Santos ES, Braulke T, Pohl S*, Schwartz IVD* (2017) Int J Biochem Cell Biol 92: 90-94Next-generation sequencing corroborates a probable de novo GNPTG variation previously detected by Sanger sequencing
Ludwig NF, Sperb-Ludwig F, Velho RV, Schwartz IVD (2017) Mol Genet Metab Rep 11: 92-99Site-1 protease and lysosomal homeostasis (Review)
Velho RV, De Pace R, Klünder S, Di Lorenzo G, Schweizer M, Braulke T, Pohl S (2017) Biochim Biophys Acta Mol Cell Res 1864: 2162-21682016
Identification of the interaction domains between α- and γ-subunits of GlcNAc-1-phosphotransferase
Velho RV, De Pace R, Tidow H, Braulke T, Pohl S (2016) FEBS Lett 590: 4287-4295Enigmatic in vivo GlcNAc-1-phosphotransferase (GNPTG) transcript correction to wild type in two mucolipidosis III gamma siblings homozygous for nonsense mutations
Velho RV, Ludwig NF, Alegra T, Sperb-Ludwig F, Guarany NR, Matte U, Schwartz IV. J Hum Genet 61: 555-5602015
Analyses of disease-related GNPTAB mutations define a novel GlcNAc1-phosphotransferase interaction domain and an alternative site-1 protease cleavage site
Velho RV, De Pace R, Klünder S, Sperb-Ludwig F, Lourenço CM, Schwartz IV, Braulke T, Pohl S (2015) Hum Mol Genet 24: 3497-3505Site-1 protease-activated formation of lysosomal targeting motifs is independent of the lipogenic transcription control
Klünder S, Heeren J, Markmann S, Santer R, Braulke T, Pohl S (2015) J Lipid Res 56: 1625-1632Subunit interactions of the disease-related hexameric GlcNAc-1-phosphotransferase complex
De Pace R, Velho RV, Encarnação M, Marschner K, Braulke T, Pohl S (2015) Hum Mol Genet 24: 6826-6835Biosynthesis, targeting, and processing of lysosomal proteins: pulse-chase labeling and immune precipitation
Pohl S, Hasilik A (2015) Methods Cell Biol 126: 63-832014
Mucolipidosis II-related mutations inhibit the exit from the endoplasmic reticulum and proteolytic cleavage of GlcNAc-1-phosphotransferase precursor protein (GNPTAB)
De Pace R, Coutinho MF, Koch-Nolte F, Haag F, Prata MJ, Alves S, Braulke T, Pohl S (2014) Hum Mutat 35:368-3762012
A novel mannose 6-phosphate specific antibody fragment for diagnosis of Mucolipidosis type II and III (Book chapter)
Pohl S, Braulke T, Müller-Loennies S (2012) In: Anticarbohydrate antibodies - From molecular basis to clinical application (Eds: P. Kosma, S. Müller-Loennies), Springer-Verlag, WienLysosomal dysfunction causes neurodegeneration in mucolipidosis II ‘knock-in’ mice Kollmann K, Damme M, Markmann S, Morelle W, Schweizer M, Hermans-Borgmeyer I, Röchert AK, Pohl S, Lübke T, Michalski JC, Käkelä R. Walkley SU, Braulke T (2012) Brain 135: 2661-2675
Multiple Enzyme Deficiencies: Defects in transport: Mucolipidosis II alpha/beta; mucolipidosis III alpha/beta and mucolipidosis III gamma (Book chapter)
Raas-Rothschild A, Pohl S, Braulke T (2012) In: Lysosomal Storage Diseases: A Practical Guide (Eds. A. B. Mehta, B. Winchester), Wiley-Blackwell, Oxford2011
A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism
Marschner K, Kollmann K, Schweizer M, Braulke T, Pohl S (2011) Science 333: 87-90Analysis of potential biomarkers and modifier genes affecting the clinical course of CLN3 disease
Lebrun AH, Moll-Khosrawi P, Pohl S, Makrypidi G, Storch S, Kilian D, Streichert T, Otto B, Mole SE, Ullrich K, Cotman S, Kohlschütter A, Braulke T, Schulz A (2011) ) Mol Med 17: 1253-1261Residual activity and proteasomal degradation of p.Ser298Pro sulfamidase identified in patients with a mild clinical phenotype of Sanfilippo A syndrome
Muschol N, Pohl S, Meyer A, Gal A, Ullrich K, Braulke T (2011) Am J Med Genet A 155: 1634-1639Post-translational modifications of the gamma-subunit affect intracellular trafficking and complex assembly of GlcNAc-1-phosphotransferase
Encarnação M, Kollmann K, Trusch M, Braulke T, Pohl S (2011) J Biol Chem 286: 5311-53182010
Proteolytic processing of the gamma-subunit is associated with the failure to form GlcNAc-1-phosphotransferase complexes and mannose 6-phosphate residues on lysosomal enzymes in human macrophages
Pohl S, Tiede S, Marschner K, Encarnação M, Castrichini M, Kollmann K, Muschol N, Ullrich K, Müller-Loennie S, Braulke T (2010) J Biol Chem 285: 23936-23944Mannose phosphorylation in health and disease (Review)
Kollmann K, Pohl S, Marschner K, Encarnação M, Sakwa I, Tiede S, Poorthuis BJ, Lübke T, Müller-Loennies S, Storch S, Braulke T (2010) Eur J Cell Biol 89: 117-123Loss of N-acetylglucosamine-1-phosphotransferase gamma-subunit due to intronic mutation in GNPTG causes mucolipidosis type III gamma: Implications for molecular and cellular diagnostics
Pohl S, Encarnacão M, Castrichini M, Müller-Loennies S, Muschol N, Braulke T (2010) Am J Med Genet A 152: 124-1322009
Compensatory expression of human N-acetylglucosaminyl-1 phosphotransferase subunits in mucolipidosis type III gamma
Pohl S, Tiede S, Castrichini M, Cantz M, Gieselmann V, Braulke T (2009) Biochim Biophys Acta 1792: 221-225Glycosylation- and phosphorylation-dependent intracellular transport of lysosomal hydrolases (Review)
Pohl S, Marschner K, Storch S, Braulke T (2009) Biol Chem 390:521-7Identification and molecular characterization of six novel mutations in the UDP-N-acetylglucosamine-1-phosphotransferase gamma subunit (GNPTG) gene in patients with mucolipidosis III gamma
Persichetti E, Chuzhanova NA, Dardis A, Tappino B, Pohl S, Thomas NS, Rosano C, Balducci C, Paciotti S, Dominissini S, Montalvo AL, Sibilio M, Parini R, Rigoldi M, Di Rocco M, Parenti G, Orlacchio A, Bembi B, Cooper DN, Filocamo M, Beccari T (2009) Hum Mutat 30: 978-9842008
Molecular analysis of the GlcNac-1-phosphotransferase (Review)
Braulke T, Pohl S, Storch S (2008) J Inherit Metab Dis 31: 253-2572007
C-terminal prenylation of the CLN3 membrane glycoprotein is required for efficient endosomal sorting to lysosomes
Storch S, Pohl S, Quitsch A, Falley K, Braulke T (2007) Traffic 8: 431-44Increased expression of lysosomal acid phosphatase (LAP/Acp2) in CLN3-defective cells and mouse brain tissue
Pohl S, Mitchison HM, Kohlschütter A, van Diggelen, O, Braulke T, Storch S (2007) J Neurochem 103: 2177-21882004
A dileucine motif and a cluster of acidic amino acids in the second cytoplasmic domain of the Batten disease-related CLN3 Protein are required for efficient lysosomal targeting
Storch S, Pohl S, Braulke T (2004) J Biol Chem 279: 53625-53634
-
Funding
Current Funding

2024-2028
German Research Foundation (DFG)
Clinical Research Group CRU 5029:
"ProBone, Precision Medicine for Early-Onset Low Bone Mineral Density Disorders"
2023 - 2024
German Research Foundation (DFG)
Research Grant „The molecular role of site-2 protease for osteblast function“
2023 - 2025
Werner Otto Stiftung
Research Grant „Distinct roles of site-1 and site-2 proteases in the pathogenesis of rare inherited connective tissue diseases of childhood“
2022 - 2026
German Research Foundation (DFG)
Heisenberg Grant „Pathophysiology and molecular bases of skeletal disorders in childhood“
2018 - 2025
German Research Foundation (DFG)
Research Grant „Role of the gamma-subunit of the GlcNAc-1-phosphotransferase complex for selective mannose 6-phosphate modifications on lysosomal enzymes“Former Funding

2010 - 2022
German Research Foundation (DFG)
Collaborative Research Centre 877:
"Proteolysis as a Regulatory Event in Pathophysiology"
www.uni-kiel.de/Biochemie/sfb877/
2017 - 2018
ISMRD - International Society for Mannosidosis & Related Diseases (USA)
"Osteoporosis in Mucolipidosis II - A potential corrective approach"
http://www.ismrd.org/research
2012 - 2017
German Research Foundation (DFG)
Research Training Group 1459:
"Sorting and Interactions between Proteins of Subcellular Compartments" -
-
Open Positions
Bachelor’s and Master’s students are an integral part of our research team. We offer Bachelor’s and Master’s thesis research projects that focus on the pathomechanisms of skeletal diseases.
We are also looking for student assistants to support our research.
We always welcome excellent MD and PhD students and postdoctoral researchers who are interested in our research and are willing to apply for scholarships to join our lab.
We also invite medical students of UKE to do their student studies (‘Studienarbeit’) on a topic related to bone and/or cartilage pathologies as well as molecular pathomechanisms of these diseases and current therapeutic strategies to combat them.
Please send your requests to Prof. Dr. Sandra Pohl (s.pohl@uke.de).

