 Research at the UKE?
Research at the UKE?

Welcome to the
Hamburg Center for Translational Immunology (HCTI)
Through translational research, we bridge lab discoveries and clinical applications, driving progress in diagnostics, therapies, and medical treatments.
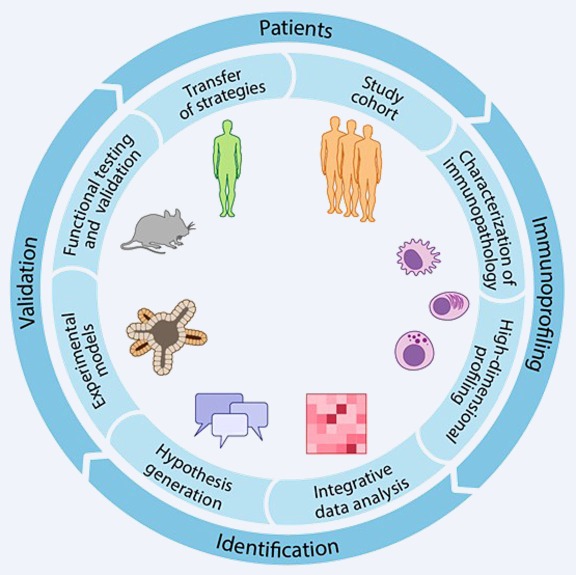
We envision a future where an interdisciplinary approach, bridging the gap between laboratory research and clinical care holds the key to unlocking innovative solutions and improving patient outcomes
Board members




Laboratories & Projects
Explore our captivating laboratories and cutting-edge research projects, where our teams address scientific questions with enthusiasm and drive forward translational research.
Events
Find details on upcoming events, including our regular Monday Seminar & the HCTI Lecture Series.
Recent selected publications
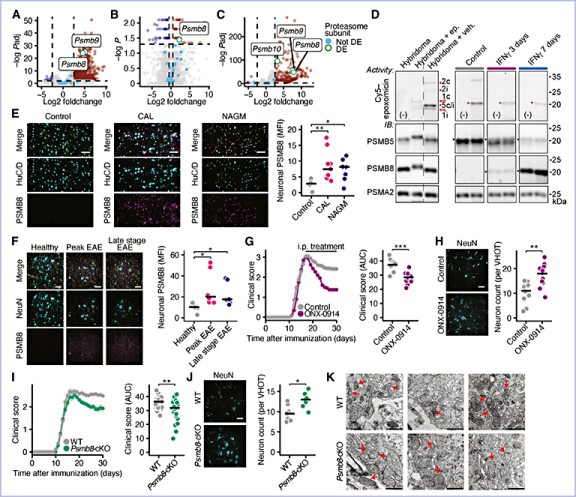
Abstract
Inflammation, aberrant proteostasis, and energy depletion are hallmarks of neurodegenerative diseases such as multiple sclerosis (MS). However, the interplay between inflammation, proteasomal dysfunction in neurons, and its consequences for neuronal integrity remains unclear. Using transcriptional, proteomic, and functional analyses of proteasomal subunits in inflamed neurons, we found that interferon-γ-mediated induction of the immunoproteasome subunit, proteasome 20S beta 8 (PSMB8) impairs the proteasomal balance, resulting in reduced proteasome activity. This reduction causes the accumulation of phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3), a key metabolic regulator, leading to enhanced neuronal glycolysis, reduced pentose phosphate pathway activity, oxidative injury, and ferroptosis. Neuron-specific genetic and systemic pharmacological targeting of PSMB8 or PFKFB3 protected neurons in vitro and in a mouse model of MS. Our findings provide a unifying explanation for proteasomal dysfunction in MS and possibly other neurodegenerative diseases, linking inflammation to metabolic disruption, and presenting an opportunity for targeted neuroprotective therapies.
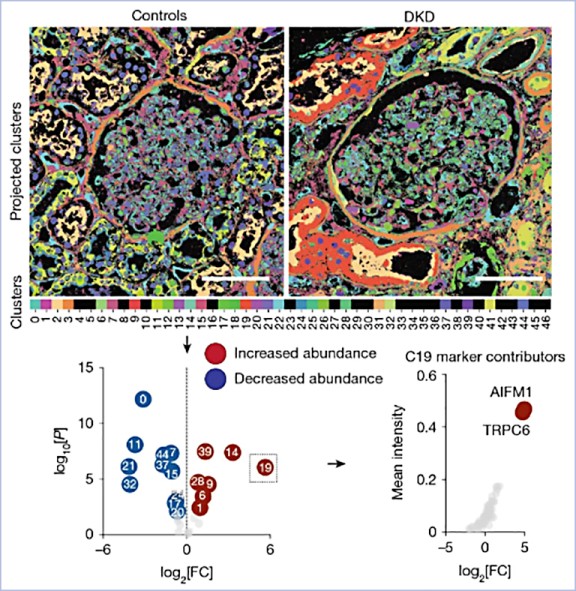
Abstract
The expression and location of proteins in tissues represent key determinants of health and disease. Although recent advances in multiplexed imaging have expanded the number of spatially accessible proteins, the integration of biological layers (that is, cell structure, subcellular domains and signalling activity) remains challenging. This is due to limitations in the compositions of antibody panels and image resolution, which together restrict the scope of image analysis. Here we present pathology-oriented multiplexing (PathoPlex), a scalable, quality-controlled and interpretable framework. It combines highly multiplexed imaging at subcellular resolution with a software package to extract and interpret protein co-expression patterns (clusters) across biological layers. PathoPlex was optimized to map more than 140 commercial antibodies at 80 nm per pixel across 95 iterative imaging cycles and provides pragmatic solutions to enable the simultaneous processing of at least 40 archival biopsy specimens. In a proof-of-concept experiment, we identified epithelial JUN activity as a key switch in immune-mediated kidney disease, thereby demonstrating that clusters can capture relevant pathological features. PathoPlex was then used to analyse human diabetic kidney disease. The framework linked patient-level clusters to organ disfunction and identified disease traits with therapeutic potential (that is, calcium-mediated tubular stress). Finally, PathoPlex was used to reveal renal stress-related clusters in individuals with type 2 diabetes without histological kidney disease. Moreover, tissue-based readouts were generated to assess responses to inhibitors of the glucose cotransporter SGLT2. In summary, PathoPlex paves the way towards democratizing multiplexed imaging and establishing integrative image analysis tools in complex tissues to support the development of next-generation pathology atlases.
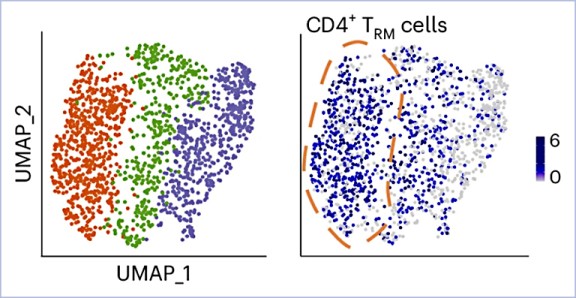
Abstract
Tissue-resident memory T (TRM) cells are a specialized T cell population that reside in tissues and provide a rapid protective response upon activation. Here, we showed that human and mouse CD4+ TRM cells existed in a poised state and stored messenger RNAs encoding proinflammatory cytokines without protein production. At steady state, cytokine mRNA translation in TRM cells was suppressed by the integrated stress response (ISR) pathway. Upon activation, the central ISR regulator, eIF2α, was dephosphorylated and stored cytokine mRNA was translated for immediate cytokine production. Genetic or pharmacological activation of the ISR–eIF2α pathway reduced cytokine production and ameliorated autoimmune kidney disease in mice. Consistent with these results, the ISR pathway in CD4+ TRM cells was downregulated in patients with immune-mediated diseases of the kidney and the intestine compared to healthy controls. Our results indicated that stored cytokine mRNA and translational regulation in CD4+ TRM cells facilitate rapid cytokine production during local immune response.
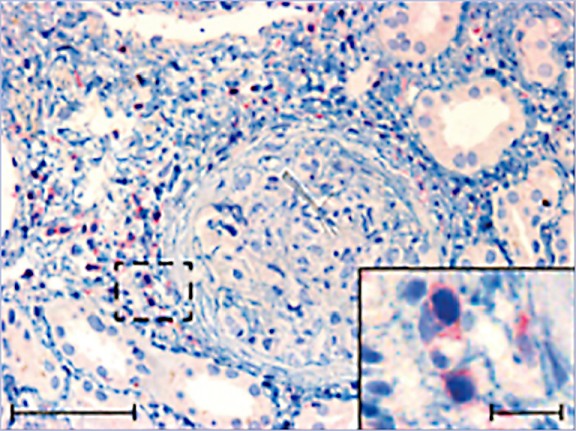
Abstract
Pro-inflammatory CD4+ T cells are major drivers of autoimmune diseases, yet therapies modulating T cell phenotypes to promote an anti-inflammatory state are lacking. Here, we identify T helper 17 (TH17) cell plasticity in the kidneys of patients with antineutrophil cytoplasmic antibody-associated glomerulonephritis on the basis of single-cell (sc) T cell receptor analysis and scRNA velocity. To uncover molecules driving T cell polarization and plasticity, we established an in vivo pooled scCRISPR droplet sequencing (iCROP-seq) screen and applied it to mouse models of glomerulonephritis and colitis. CRISPR-based gene targeting in TH17 cells could be ranked according to the resulting transcriptional perturbations, and polarization biases into T helper 1 (TH1) and regulatory T cells could be quantified. Furthermore, we show that iCROP-seq can facilitate the identification of therapeutic targets by efficient functional stratification of genes and pathways in a disease- and tissue-specific manner. These findings uncover TH17 to TH1 cell plasticity in the human kidney in the context of renal autoimmunity.
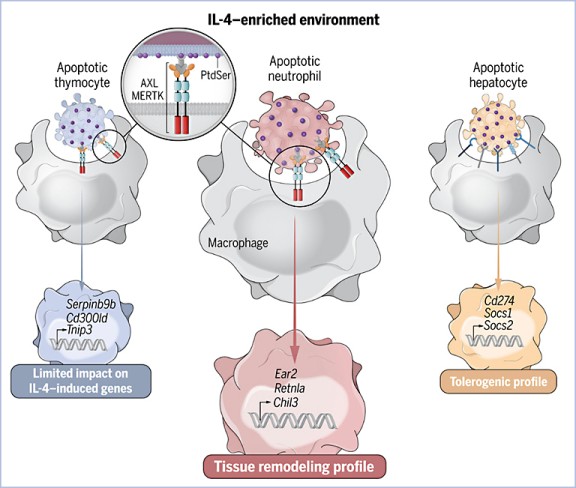
Abstract
Macrophages are functionally heterogeneous cells essential for apoptotic cell clearance. Apoptotic cells are defined by homogeneous characteristics, ignoring their original cell lineage identity. We found that in an interleukin-4 (IL-4)-enriched environment, the sensing of apoptotic neutrophils by macrophages triggered their tissue remodeling signature. Engulfment of apoptotic hepatocytes promoted a tolerogenic phenotype, whereas phagocytosis of T cells had little effect on IL-4-induced gene expression. In a mouse model of parasite-induced pathology, the transfer of macrophages conditioned with IL-4 and apoptotic neutrophils promoted parasitic egg clearance. Knockout of phagocytic receptors required for the uptake of apoptotic neutrophils and partially T cells, but not hepatocytes, exacerbated helminth infection. These findings suggest that the identity of apoptotic cells may contribute to the development of distinct IL-4-driven immune programs in macrophages.